
Tesla’s been undergoing some major changes, and now we have a sense of why: The company says it is upending...



























Any day now, people will look up and see a distant star exploding. Though Earthlings will be able to observe this celestial event as if it...


The ground is moving. It’s a creeping movement, but a momentous one. Some 200 million years ago, a single, extraordinary supercontinent called Pangea dominated Earth. Ultimately,...


With The Jinx: Part Two coming to Max, you might be scratching your brain trying to recall all the relevant details unfurled in the groundbreaking true...
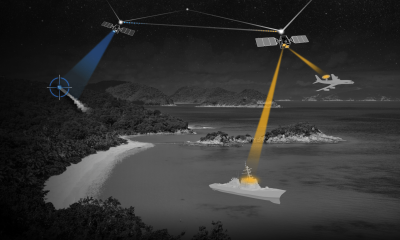

CesiumAstro alleges in a newly filed lawsuit that a former executive disclosed trade secrets and confidential information about sensitive tech, investors and customers to a competing...


“God, if I could finally get a big Le Creuset, things might really turn around for me.” It’s a lofty, quick fix pitched by Nicola Coughlan’s...